Solutions
In a world of unlimited resources there would be no need to worry about efficiently and economically collecting samples for answering a particular question. However, time and money are always finite, particularly in the areas of wildlife and conservation. Accordingly, at Pisces we work hard to make sure a client’s limited resources are efficiently utilized. The following are a series of stories illustrating some of the solutions we have come up with over the years.
Discuss your next project

Environmental DNA sample collection
While the basic requirements of environmental DNA (eDNA) sample collection are simple – pass water (or air) through a micron pore-size filter – a wide variety of apparatuses have been developed. Many of these are complicated (peristaltic pumps, filter funnels and flasks), heavy (rechargeable batteries), expensive (Smith-Root eDNA Sampler Backpack)...
Read More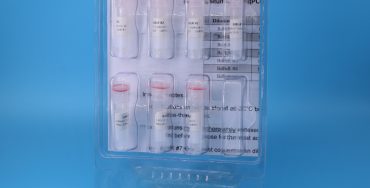
Plasmid-based qPCR positive controls
Over time, qPCR assays have steadily superseded PCR assays, both for their ability to provide quantitation of target copy number, as well as decreasing the risk of carry-forward contamination since there is no need to open reaction tubes containing amplified DNA. However, one of the requirements for qPCR assays is...
Read More
“Instant” temporal studies from archived DNA samples
Right from Pisces’ beginning we have archived all sample DNA extracts. Our typical spin-column DNA extraction procedure gives a final volume of DNA extract of 200 µL. A PCR or qPCR reaction uses 2 to 4 µL of DNA extract, so even testing in triplicate (the standard for eDNA samples)...
Read More

Testing multi-worm samples of Tubifex
As a part of their work on Trout Whirling Disease, Beauchamp, et al. determined that there were four sub-species or lineages of Tubifex tubifex, the alternate host for Myxobolus cerebralis, and that the lineages had different sensitivity to infection by M.c. (Beauchamp, et al., 2002). Since this differential susceptibility might...
Read More
Skin swab pooling for chytrid testing
In the early days of chytrid fungus testing (ca. 2007), there was a need for relatively large-scale screening of locations or amphibian populations for the presence or absence of Batrachochytrium dendrobatidis. At the time, this could only be done by collecting and testing skin swab samples from individual animals –...
Read More
